Your healthcare professional should have directed you to this website for general education information about sotatercept. This information does not replace your healthcare professional’s advice.
Please always follow your healthcare professional’s instructions and talk with them about any questions or problems you have regarding your health and treatment.
All the information on this website is for adults, not children or adolescents. For information for children and adolescents, please consult with your healthcare professional for more information.
Indication
WINREVAIR® (sotatercept), in combination with other pulmonary arterial hypertension (PAH) therapies, is indicated for the treatment of PAH in adult patients with World Health Organization (WHO) Functional Class (FC) II to III, to improve exercise capacity.1,2
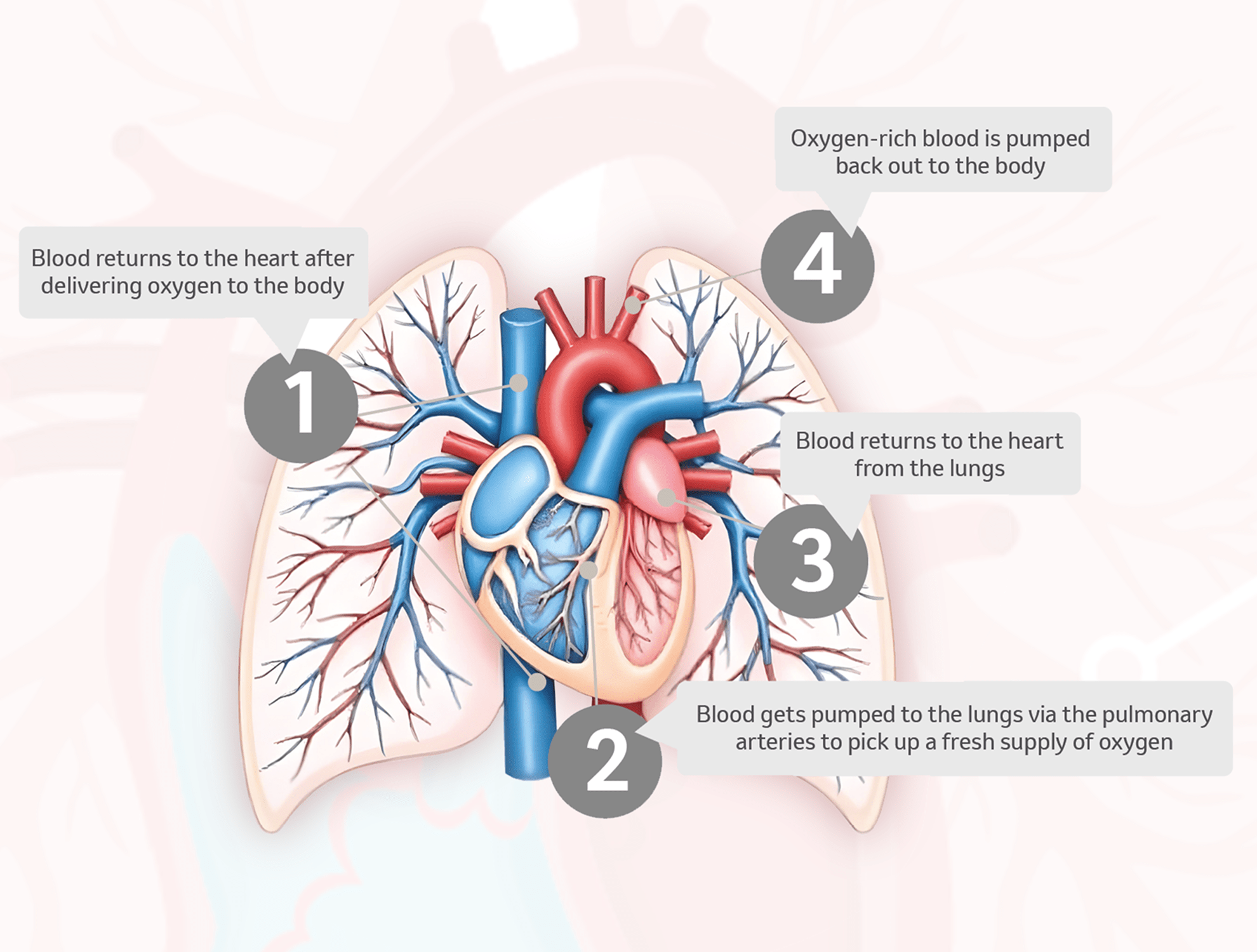
How does Sotatercept work?
Sotatercept is the first and only activin signalling inhibitor indicated for PAH treatment.1,2
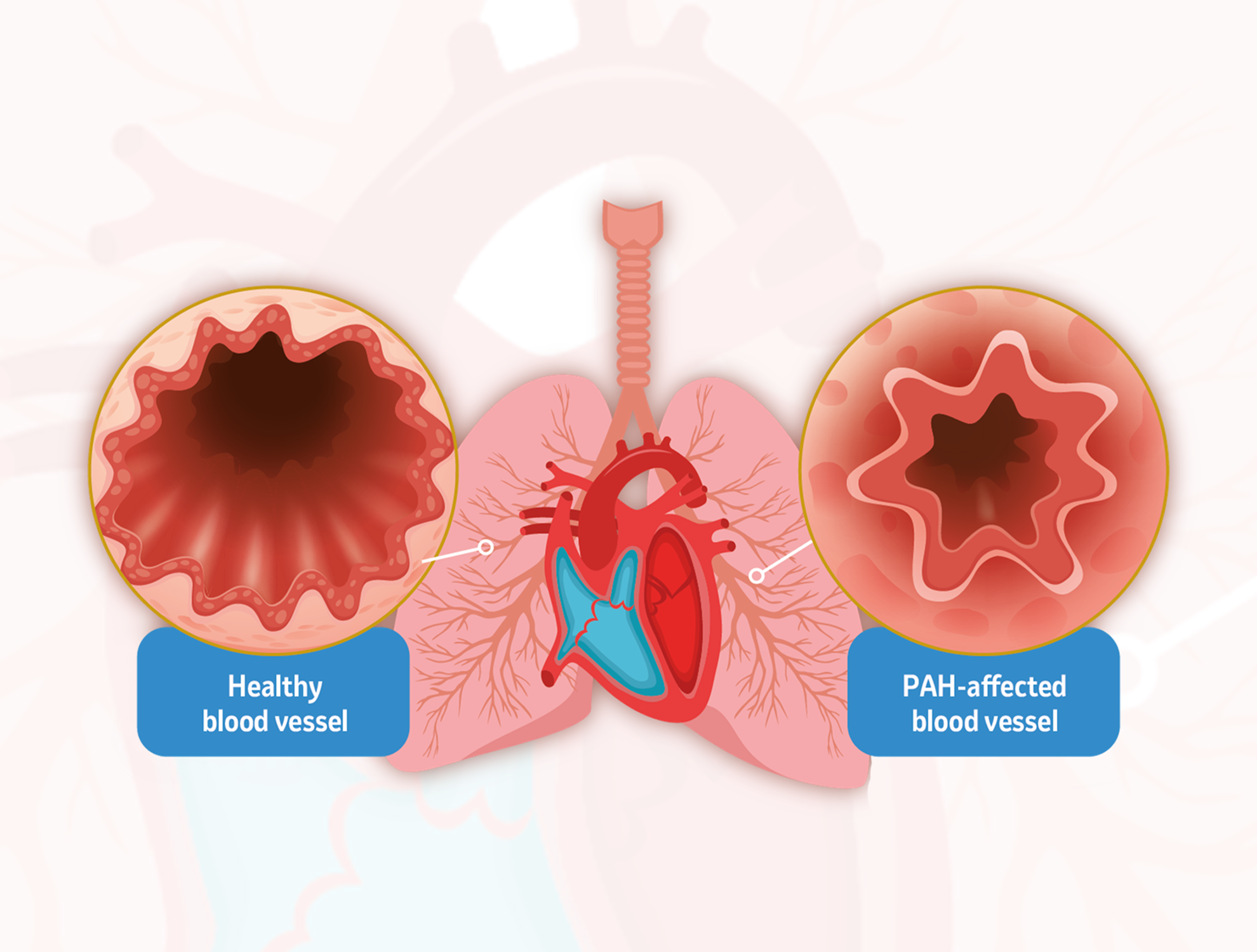
In patients with PAH, signalling from excessive activins, a type of protein, leads to thickening of the artery walls.5
Sotatercept is designed to bind to activins, thereby reducing their signalling, and decrease excessive cell division, ultimately aiming to target a key cause of PAH progression.5

Watch the full mechanism of action video here:
Step-by-step self-administration video
Before administering Sotatercept, watch the self-administration video below and refer to the Instructions for Use Booklet.
Possible side effects6
Like all medicines, this medicine can cause side effects.
Talk to your doctor or pharmacist immediately if you notice:
- Easy bruising, prolonged bleeding from cuts and nosebleeds. These could be signs of a low number of platelets (thrombocytopenia). This will be shown in your blood tests.
In addition, your doctor will do regular blood tests to notice whether you have:
- High levels of haemoglobin
The serious side effects above may affect more than 1 in 10 people.
Talk to your doctor or pharmacist if you notice any of the following:
-
Very common (may affect more than 1 in 10 people)
Headache, Nosebleeds (epistaxis), Spider veins or tiny blood vessels that look like pink or red lines on the skin (telangiectasia), Diarrhoea, Dizziness, Skin rash
-
Common (may affect up to 1 in 10 people)
High blood pressure, Redness of the skin, Bleeding gums, Itching at the injection site
Additional warnings1,2
Fertility: Based on findings in animals, Sotatercept may impair female and male fertility.
Children: The safety and efficacy of Sotatercept in children and adolescents below 18 years of age has not yet been established.
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Let your PH specialist team know about all medications you are currently taking, including any prescription drugs, vitamins, minerals, natural supplements, or alternative treatments. It is not known if Sotatercept interacts with other medications or supplements.
References
- Sotatercept (45 mg) Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/product/100843/smpc.
- Sotatercept (60 mg) Summary of Product Characteristics. Available from: https://www.medicines.org.uk/emc/product/100844/smpc.
- What is PH? PHA UK. Available at: https://www.phauk.org/about-ph-2/what-is-ph/.
- Lan NS, et al. Diseases. 2018 May 16;6(2):38.
- Humbert M, et al. N Engl J Med. 2021;384(13);1204-15.
- Sotatercept. Patient Information Leaflet. 2025. Available from: https://www.medicines.org.uk/emc/product/100844/pil.